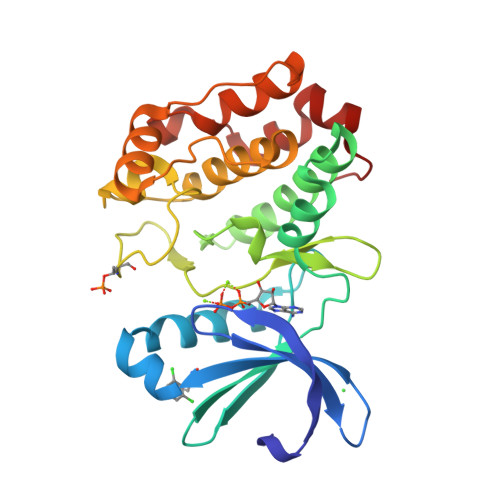
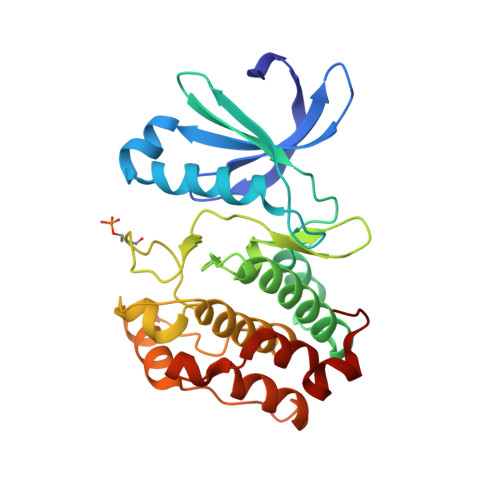
Characterization of Three Druggable Hot-Spots in the Aurora-A/TPX2 Interaction Using Biochemical, Biophysical, and Fragment-Based Approaches.
McIntyre, P.J., Collins, P.M., Vrzal, L., Birchall, K., Arnold, L.H., Mpamhanga, C., Coombs, P.J., Burgess, S.G., Richards, M.W., Winter, A., Veverka, V., Delft, F.V., Merritt, A., Bayliss, R.(2017) ACS Chem Biol 12: 2906-2914
- PubMed: 29045126
- DOI: https://doi.org/10.1021/acschembio.7b00537
- Primary Citation of Related Structures:
5ORL, 5ORN, 5ORO, 5ORP, 5ORR, 5ORS, 5ORT, 5ORV, 5ORW, 5ORX, 5ORY, 5ORZ, 5OS0, 5OS1, 5OS2, 5OS3, 5OS4, 5OS5, 5OS6, 5OSD, 5OSE, 5OSF - PubMed Abstract:
The mitotic kinase Aurora-A and its partner protein TPX2 (Targeting Protein for Xenopus kinesin-like protein 2) are overexpressed in cancers, and it has been proposed that they work together as an oncogenic holoenzyme. TPX2 is responsible for activating Aurora-A during mitosis, ensuring proper cell division. Disruption of the interface with TPX2 is therefore a potential target for novel anticancer drugs that exploit the increased sensitivity of cancer cells to mitotic stress. Here, we investigate the interface using coprecipitation assays and isothermal titration calorimetry to quantify the energetic contribution of individual residues of TPX2. Residues Tyr8, Tyr10, Phe16, and Trp34 of TPX2 are shown to be crucial for robust complex formation, suggesting that the interaction could be abrogated through blocking any of the three pockets on Aurora-A that complement these residues. Phosphorylation of Aurora-A on Thr288 is also necessary for high-affinity binding, and here we identify arginine residues that communicate the phosphorylation of Thr288 to the TPX2 binding site. With these findings in mind, we conducted a high-throughput X-ray crystallography-based screen of 1255 fragments against Aurora-A and identified 59 hits. Over three-quarters of these hits bound to the pockets described above, both validating our identification of hotspots and demonstrating the druggability of this protein-protein interaction. Our study exemplifies the potential of high-throughput crystallography facilities such as XChem to aid drug discovery. These results will accelerate the development of chemical inhibitors of the Aurora-A/TPX2 interaction.
Organizational Affiliation:
Department of Molecular and Cell Biology, Henry Wellcome Building, University of Leicester , Leicester, LE1 9HN, United Kingdom.