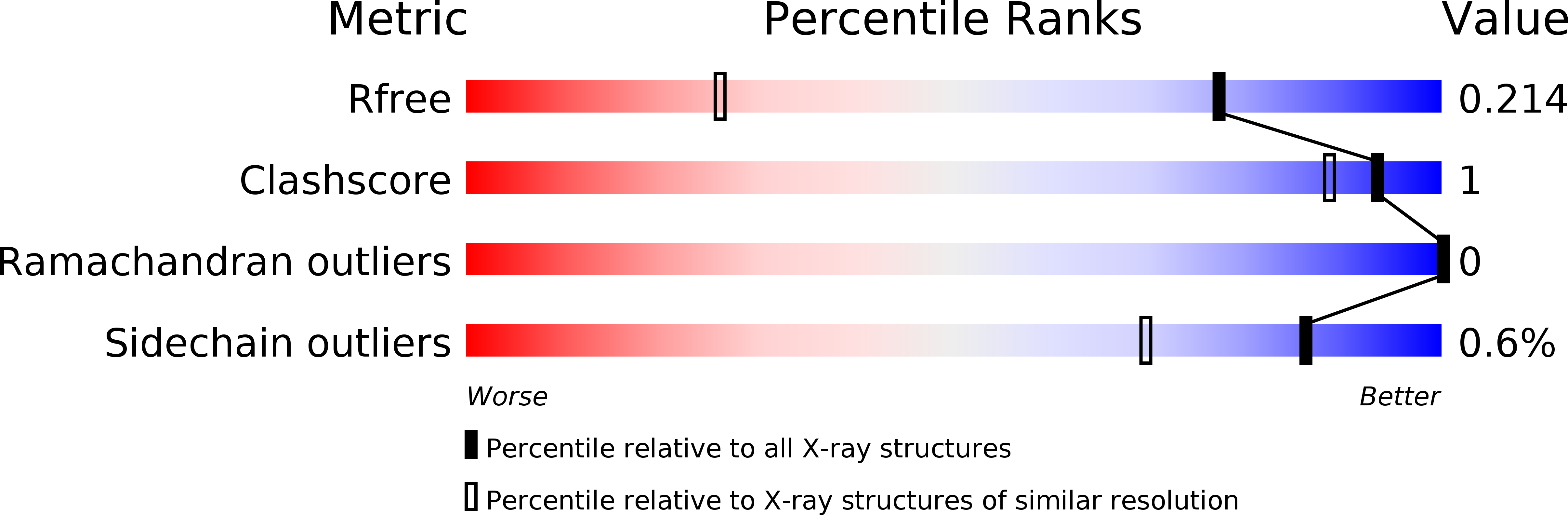
Structure-Activity Relationships ofcyclo(l-Tyrosyl-l-tyrosine) Derivatives Binding toMycobacterium tuberculosisCYP121: Iodinated Analogues Promote Shift to High-Spin Adduct.
Rajput, S., McLean, K.J., Poddar, H., Selvam, I.R., Nagalingam, G., Triccas, J.A., Levy, C.W., Munro, A.W., Hutton, C.A.(2019) J Med Chem 62: 9792-9805
- PubMed: 31618032
- DOI: https://doi.org/10.1021/acs.jmedchem.9b01199
- Primary Citation of Related Structures:
6RQ0, 6RQ1, 6RQ3, 6RQ5, 6RQ6, 6RQ8, 6RQ9, 6RQB, 6RQD, 6RQE - PubMed Abstract:
A series of analogues of cyclo (l-tyrosyl-l-tyrosine), the substrate of the Mycobacterium tuberculosis enzyme CYP121, have been synthesized and analyzed by UV-vis and electron paramagnetic resonance spectroscopy and by X-ray crystallography. The introduction of iodine substituents onto cyclo (l-tyrosyl-l-tyrosine) results in sub-μM binding affinity for the CYP121 enzyme and a complete shift to the high-spin state of the heme Fe III . The introduction of halogens that are able to interact with heme groups is thus a feasible approach to the development of next-generation, tight binding inhibitors of the CYP121 enzyme, in the search for novel antitubercular compounds.
Organizational Affiliation:
School of Chemistry and Bio21 Molecular Science and Biotechnology Institute , University of Melbourne , 30 Flemington Road , Parkville , Victoria 3010 , Australia.