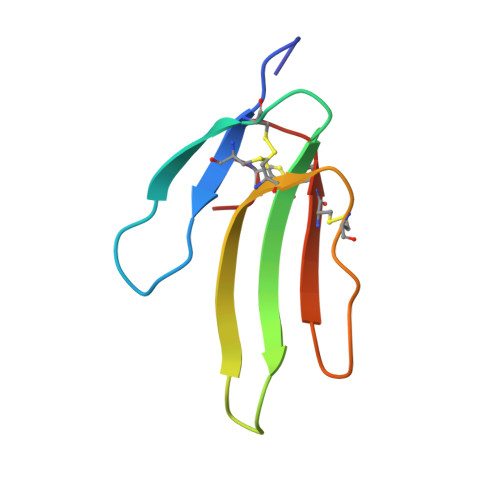
Structure and selectivity engineering of the M1muscarinic receptor toxin complex.
Maeda, S., Xu, J., N Kadji, F.M., Clark, M.J., Zhao, J., Tsutsumi, N., Aoki, J., Sunahara, R.K., Inoue, A., Garcia, K.C., Kobilka, B.K.(2020) Science 369: 161-167
- PubMed: 32646996
- DOI: https://doi.org/10.1126/science.aax2517
- Primary Citation of Related Structures:
6WJC - PubMed Abstract:
Muscarinic toxins (MTs) are natural toxins produced by mamba snakes that primarily bind to muscarinic acetylcholine receptors (MAChRs) and modulate their function. Despite their similar primary and tertiary structures, MTs show distinct binding selectivity toward different MAChRs. The molecular details of how MTs distinguish MAChRs are not well understood. Here, we present the crystal structure of M 1 AChR in complex with MT7, a subtype-selective anti-M 1 AChR snake venom toxin. The structure reveals the molecular basis of the extreme subtype specificity of MT7 for M 1 AChR and the mechanism by which it regulates receptor function. Through in vitro engineering of MT7 finger regions that was guided by the structure, we have converted the selectivity from M 1 AChR toward M 2 AChR, suggesting that the three-finger fold is a promising scaffold for developing G protein-coupled receptor modulators.
Organizational Affiliation:
Department of Molecular and Cellular Physiology, Stanford University School of Medicine, Stanford, CA 94305, USA. [email protected] [email protected].